What Pairs of Elements Form Ionic Compounds
Two-element ionic compounds include sodium chloride or table salt. C chlorine and bromine.
Examples of ionic compound pairs are sodium chlorideNaclPotassium iodide KI magnesium chloride Mgcl2 sodium hydrideNaH Iron II chloride Fecl2.

. A fluorine and sodium B oxygen and magnesium C potassium and sulfur D iron and chlorine E. Assign each species to the expression that most accurately. Determine whether the following pairs of elements can form ionic compounds.
Potassium and sulfur helium and oxygen magnesium and chlorine sodium and potassium nitrogen and iodine chlorine and bromine. Of the choices shown Zn and O are the most likely to form an ionic compound ZnO. Please not the attached picture is not the correct answer.
Identify the following as ionic or covalent bonds. K 2 O. Which of the following pairs of elements are likely to form ionic compounds.
Ionic compounds are defined as the compounds in which there is complete transfer of electrons from one element a metal to another element a non-metal. F nitrogen and oxygen. Note that ionic compounds formed are functions of the valencies of the metals and nonmetals.
Usually a metallic element and a non metallic element form an ionic compound but. Topic 6 - Kinetics. Ionic compounds are formed by the reaction of metal atoms with non-metal atoms.
The opposite charges attract and the oppositely charged ions form an ionic bond. 2 lithium and fluorine. E nitrogen and iodine.
Terms in this set 23 Determine whether the following pairs of elements can form ionic compounds. E sodium and potassium. C potassium and sulfur.
A fluorine and sodium B oxygen and magnesium C potassium and sulfur D iron and chlorine. Ionic compounds generally form between elements that are metals and elements that are nonmetals. Ionic Compounds involves strong electrostatic forces.
B magnesium and chlorine. The determine-whether-the-following-pairs-of-elements-can-form-ionic-compounds-sodium-and-oxygen-potassium-and-calcium-nitrogen have 1136 and 14. Which of the following pairs of elements are likely to form ionic compounds.
D potassium and sulfur. Ionic compound is formed by the elements. What are the kinds of elements that combine to form ionic compounds are what.
In other words the element symbol for the metal is. Molecular compound D Molecular elements E Ionic compound F Atomic elements G Ionic compound H Atomic elements. B one atom of sodium and one atom of fluorine will form an ionic bond since ionic bonds are combinations of a metal and a non metal element.
A Sodium and Magnesium b Oxygen and Magnesium c Fluorine and Sodium. The ionic bonds are generally formed between a metal and a nonmetal. View Available Hint s Reset Help nuorine and sodium DO Part A Determine whether the following pairs of elements can form ionic compounds View Available Hint s Reset Help potassium and.
Ionic compounds are formed by electrostatic attraction between a cation metal ion and anion non-metal ion of similar size. In this compound there are two negative chloride ions for each positive calcium ion. Ionic bonds are the weaker electrostatic interactions formed by the transfer of electrons in the charged elemental species.
The number of pairs of electrons in a covalent bond equals the bond order. View the full answer. Details of Determine whether the following pairs of elements can form ioniccompounds Ionic or Not Ionic1- ir MP3 check it out.
D iron and chlorine. A Sodium and Magnesium b Oxygen and Magnesium c Fluorine and Sodium d Sulfur and Bromine e Manganese and Chlorine f Potassium and Sulfur. A helium and oxygen.
Click hereto get an answer to your question Determine whether the following pairs of elements can form ionic compoundsa. Determine whether the following pairs of elements can form ionic compounds. For example the metal calcium Ca and the nonmetal chlorine Cl form the ionic compound calcium chloride CaCl2.
If the size difference is such that cation is small and anion is very large they form bond with more covalent character. Here the sodium ion N a N a is a positive ion so it is attracted to the chlorine atom ion C l C l - which has a negative charge and the ionic bond is formed. F lithium and calcium.
Note that ionic compounds are named with the cation or positively-charged atom written before the anion or negatively-charged atom. 1 aluminium and oxygen. The elemental pairs that form ionic compounds are Sodium and Oxygen Manganese and Chlorine Oxygen and Calcium and Chlorine and Sodium.
From the given elements two pairs will result in the formation of ionic compounds. Which pair of elements form an ionic compound. B oxygen and magnesium.
2 days agoAvailable here are Chapter 4 - Atomic Structure Chemical Bonding Exercises Questions with Solutions and detail explanation for your practice before the examination Jun 11 2021 Chemical bonds are the attractive forces that hold atoms together in the form of compounds. Determine whether the following pairs of elements can form ionic compounds. A fluorine and sodium.
When a metal and a nonmetal exchange electrons to make a cation and an anion respectively they can form an ionic compound. Determine whether the following pairs of elements can form ionic compounds.
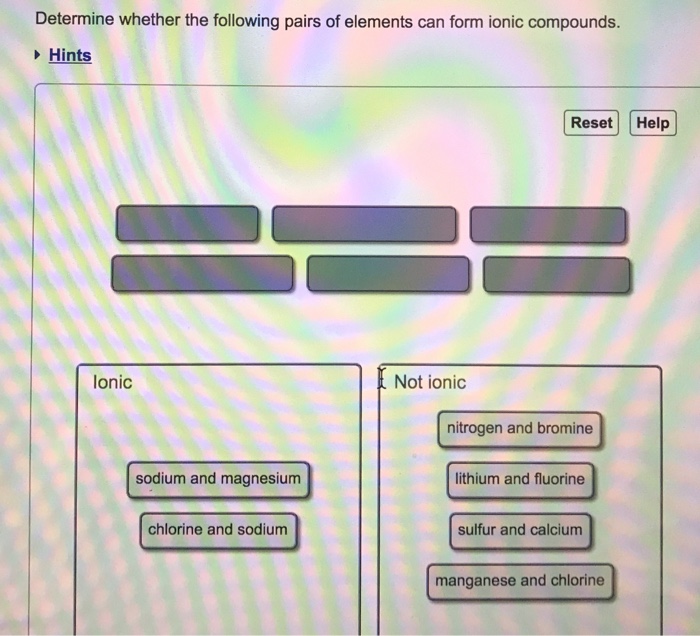
Solved Determine Whether The Following Pairs Of Elements Can Chegg Com
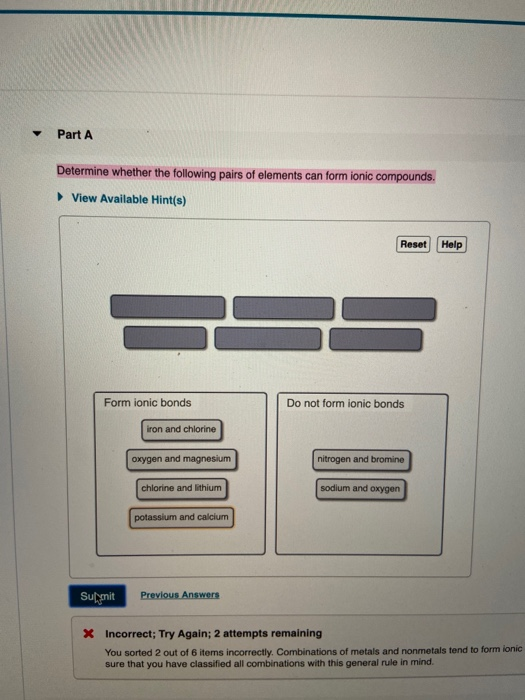
Solved Part A Determine Whether The Following Pairs Of Chegg Com

Which Of The Following Pairs Of Elements Could React To Form An Ionic Compound Check All That Apply Brainly Com

Solved Which Of The Following Pairs Of Elements Are Likely Chegg Com
No comments for "What Pairs of Elements Form Ionic Compounds"
Post a Comment